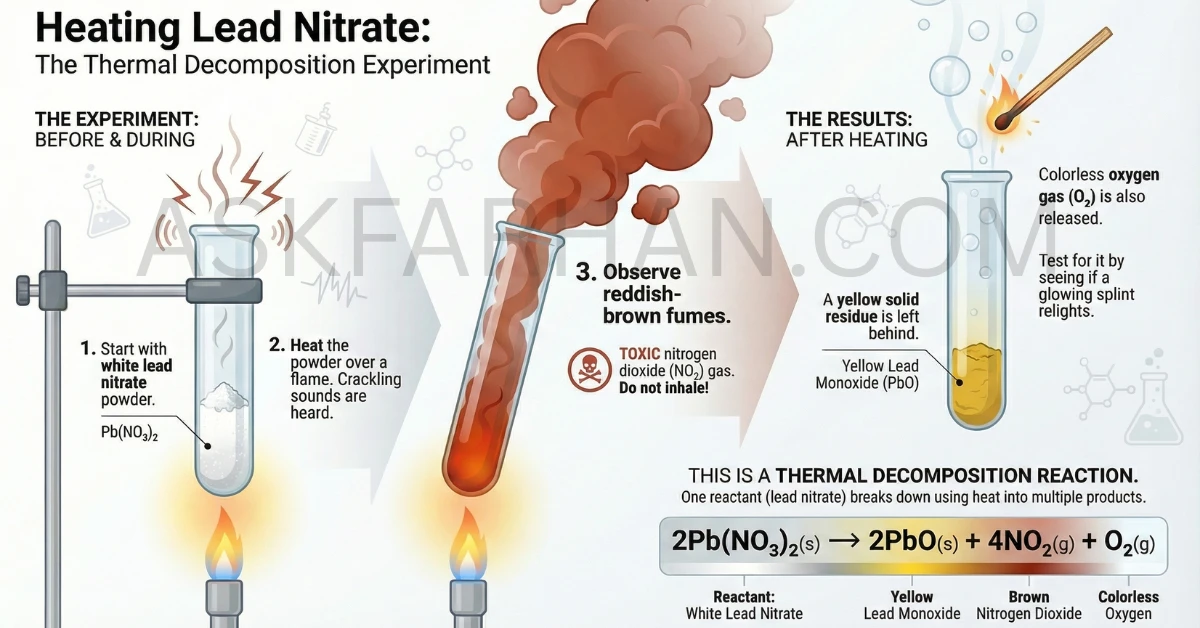
Activity 1.6: Thermal Decomposition of Lead Nitrate | Class 10 Science Ch 1
This classic chemistry experiment demonstrates one of the most visually striking examples of thermal decomposition. When you heat lead nitrate powder, you’ll witness fascinating color changes, gas evolution, and residue formation—all crucial observations for understanding decomposition reactions in your Class 10 board exams.
EXAM TIP: Looking for the Perfect Answer to Write in Your Exam?
Skip the long explanation and get straight to the exam-ready answer format with proper observations, chemical equation, and marking scheme breakdown for this 3-mark activity question!
📝 Jump to Exam-Ready Answer (3 Marks) →⚡ Quick Answer
Observations: When lead nitrate is heated, it undergoes thermal decomposition producing a yellow residue (lead monoxide), brown fumes (nitrogen dioxide), and oxygen gas. The white crystalline powder turns yellow, crackling sounds are heard, and reddish-brown nitrogen dioxide gas is released.
🔬 Understanding Activity 1.6
Activity 1.6 from NCERT Class 10 Science is designed to help you observe and understand thermal decomposition reactions. Lead nitrate, with the chemical formula \(\text{Pb(NO}_3\text{)}_2\), is a white crystalline compound that breaks down when heated, producing multiple products that can be observed through color changes and gas evolution.
This experiment is particularly important because it demonstrates all three key aspects of a decomposition reaction: solid residue formation, gas evolution, and visible color changes—making it a favorite in board examinations.
🧪 Experimental Setup and Procedure
The experiment requires simple apparatus and careful observation:
📋 Materials Required:
- Lead nitrate powder (approximately 2 grams)
- Boiling tube (dry and clean)
- Pair of tongs
- Burner/spirit lamp
- Test tube holder (optional)
⚠️ Safety Precautions:
- Never inhale the brown fumes produced—they contain toxic nitrogen dioxide
- Hold the boiling tube with tongs at a safe angle (slightly tilted)
- Perform the experiment in a well-ventilated area
- Wear safety goggles to protect eyes from spattering
- Do not touch the heated tube immediately after the experiment
Step-by-Step Procedure:
🎨 Visual Representation of the Experiment
BEFORE HEATING:
🔥 Flame
↑
|
┌──┴──┐
│ │ ← Boiling tube held with tongs
│ │
│ ⚪ │ ← White crystalline lead nitrate powder
│ ⚪⚪ │ (Pb(NO₃)₂)
└─────┘
DURING HEATING:
🔥 Flame (continuous heating)
↑
|
┌──┴──┐ ☁️ ☁️ ← Brown fumes (NO₂)
│ ☁️ │ escaping
│ ☁️ │
│ 🟡 │ ← Color changing from white to yellow
│🟡⚪ │ Crackling sounds heard
└─────┘
AFTER HEATING:
(Fumes dispersed)
┌─────┐
│ │
│ │
│ 🟡 │ ← Yellow residue (PbO - Lead monoxide)
│ 🟡🟡│ remaining in the tube
└─────┘
Products Formed:
• Yellow solid residue → Lead monoxide (PbO)
• Brown fumes → Nitrogen dioxide (NO₂)
• Colorless gas → Oxygen (O₂) - not visible
This visual representation helps you understand the transformation during thermal decomposition
👁️ Detailed Observations
When you perform this activity carefully, you’ll notice several distinct changes. Let’s break down each observation:
1. Color Change 🎨
Initial color: White crystalline powder
Final color: Yellow residue
Reason: Lead nitrate \(\text{Pb(NO}_3\text{)}_2\) decomposes to form yellow lead monoxide (PbO)
2. Evolution of Brown Fumes 💨
Observation: Reddish-brown colored fumes are released
Gas identified: Nitrogen dioxide \(\text{(NO}_2\text{)}\)
Important note: These fumes are toxic and should not be inhaled. This is why the experiment must be performed in a well-ventilated area.
3. Crackling Sound 🔊
Observation: Crackling or popping sounds are heard during heating
Reason: The crystalline structure of lead nitrate breaks down rapidly, and gases are released suddenly, creating these sounds.
4. Oxygen Gas Evolution (Invisible) 💨
Observation: Colorless, odorless oxygen gas is also released (though not visible)
Test: You can test for oxygen by bringing a glowing splint near the mouth of the tube—it will rekindle
Note: This is often missed by students but is an important product of the reaction.
⚗️ Chemical Equation
The thermal decomposition of lead nitrate can be represented by the following balanced chemical equation:
Balanced Chemical Equation:
Understanding the Equation:
| Component | Formula | State | Color/Appearance |
|---|---|---|---|
| Lead nitrate (Reactant) | \(\text{Pb(NO}_3\text{)}_2\) | Solid (s) | White crystalline |
| Lead monoxide (Product) | PbO | Solid (s) | Yellow residue |
| Nitrogen dioxide (Product) | \(\text{NO}_2\) | Gas (g) | Reddish-brown fumes |
| Oxygen (Product) | \(\text{O}_2\) | Gas (g) | Colorless, odorless |
💡 Key Points About the Equation:
- The coefficient 2 before \(\text{Pb(NO}_3\text{)}_2\) ensures the equation is balanced
- The symbol Δ (delta) above the arrow indicates heat is required
- This is a decomposition reaction where one reactant breaks into multiple products
- The reaction is endothermic (requires heat energy to proceed)
🔄 Type of Chemical Reaction
This activity demonstrates a thermal decomposition reaction, which is a specific type of decomposition reaction where heat energy is used to break down a compound.
✅ Characteristics of Decomposition Reactions:
- Single reactant: One compound breaks down
- Multiple products: Two or more simpler substances are formed
- Energy requirement: Usually requires heat, light, or electricity
- General form: AB → A + B
❌ Common Mistakes Students Make
Mistake #1: Incorrect Chemical Formula
Wrong: Writing lead nitrate as \(\text{PbNO}_3\) or \(\text{Pb}_2\text{NO}_3\)
Correct: \(\text{Pb(NO}_3\text{)}_2\) – Lead has +2 charge and nitrate has -1 charge, so we need two nitrate ions
Mistake #2: Missing the Oxygen Gas
Wrong: Writing only PbO and \(\text{NO}_2\) as products
Correct: Include \(\text{O}_2\) gas as well—it’s colorless so easily forgotten, but it’s there!
Mistake #3: Not Balancing the Equation
Wrong: \(\text{Pb(NO}_3\text{)}_2 \rightarrow \text{PbO} + \text{NO}_2 + \text{O}_2\)
Correct: \(2\text{Pb(NO}_3\text{)}_2 \rightarrow 2\text{PbO} + 4\text{NO}_2 + \text{O}_2\)
Always count atoms on both sides!
🔬 Lab Connection & Practical Tips
This activity is a staple in Class 10 chemistry practicals. Here’s what you need to know for performing it successfully in your lab:
🎯 Practical Exam Tips:
- Tube angle: Hold the boiling tube at a slight angle (about 45°) to prevent condensed moisture from flowing back and cracking the hot tube
- Heating technique: Move the tube gently in the flame for uniform heating—don’t keep it stationary
- Observation timing: Start noting observations as soon as you begin heating, not just at the end
- Safety first: Always mention safety precautions in your practical copy
- Residue examination: After cooling, the yellow residue can be examined—it won’t revert to white
✍️ What to Write in Your Practical Copy:
- Aim: To study the thermal decomposition of lead nitrate
- Materials required: List all apparatus and chemicals
- Procedure: Step-by-step method (4-5 points)
- Observations: All four observations mentioned above
- Chemical equation: Balanced equation with states
- Type of reaction: Thermal decomposition
- Conclusion: Lead nitrate decomposes on heating to form PbO, \(\text{NO}_2\), and \(\text{O}_2\)
- Precautions: At least 3-4 safety measures
📝 How to Write This Answer in Your Exam (3 Marks)
Model Answer for Board Exams:
Observations when lead nitrate is heated:
- Color change: The white crystalline lead nitrate powder turns into a yellow residue (lead monoxide).
- Evolution of brown fumes: Reddish-brown colored nitrogen dioxide gas \(\text{(NO}_2\text{)}\) is released.
- Crackling sound: Crackling or popping sounds are heard during heating.
- Oxygen gas: Colorless oxygen gas \(\text{(O}_2\text{)}\) is also evolved (can be tested with glowing splint).
Chemical equation:
Type of reaction: Thermal decomposition reaction
📊 Marking Scheme Breakdown (3 Marks):
- 1 mark: Correct observations (color change + brown fumes)
- 1 mark: Balanced chemical equation with correct formulas
- 1 mark: Identifying type of reaction OR mentioning all products correctly
💡 Exam Writing Tips:
- Always write observations in past tense (“was observed”, “turned yellow”)
- Use proper chemical formulas with correct subscripts and superscripts
- Don’t forget to mention the Δ symbol above the arrow in the equation
- Include state symbols (s), (g) in the equation for extra marks
- If asked for safety precautions, mention “well-ventilated area” and “do not inhale fumes”
❓ Frequently Asked Questions (FAQs)
Q1. Why does lead nitrate turn yellow when heated?
Lead nitrate decomposes to form lead monoxide (PbO), which is naturally yellow in color. The yellow color is an intrinsic property of PbO. The transformation from white \(\text{Pb(NO}_3\text{)}_2\) to yellow PbO is a clear visual indicator that a chemical reaction has occurred. This color change is permanent and the yellow residue will not turn back to white even after cooling.
Q2. What are the brown fumes produced in this reaction?
The brown fumes are nitrogen dioxide \(\text{(NO}_2\text{)}\) gas. This gas has a characteristic reddish-brown color and a pungent odor. It’s important to note that \(\text{NO}_2\) is toxic and should not be inhaled. This is why the experiment must be performed in a well-ventilated area or under a fume hood. The brown color is so distinctive that it’s often used as a test to identify nitrogen dioxide in chemical analysis.
Q3. How can we test for the presence of oxygen gas in this reaction?
Oxygen gas can be tested using the glowing splint test. Take a wooden splint, light it, then blow it out so it’s glowing (not flaming). If you bring this glowing splint near the mouth of the test tube during the reaction, the splint will rekindle (burst into flames again). This is because oxygen supports combustion. This test is a standard method to confirm the presence of oxygen gas in any chemical reaction.
Q4. Why is this called a decomposition reaction?
This is called a decomposition reaction because a single compound (lead nitrate) breaks down into two or more simpler substances (lead monoxide, nitrogen dioxide, and oxygen). Specifically, it’s a thermal decomposition reaction because heat energy is required to break the chemical bonds in lead nitrate. The general form of decomposition reactions is AB → A + B, where one reactant produces multiple products.
Q5. What precautions should be taken while performing this activity?
Several important precautions must be followed: (1) Never inhale the brown fumes as nitrogen dioxide is toxic; (2) Hold the test tube with tongs at a slight angle to prevent moisture from flowing back and cracking the hot tube; (3) Perform the experiment in a well-ventilated area or under a fume hood; (4) Wear safety goggles to protect your eyes from any spattering; (5) Do not touch the tube immediately after heating as it remains very hot; (6) Use only a small amount (about 2g) of lead nitrate to avoid excessive fume production.
✍️ Written by Dr. Irfan Mansuri
Senior Science Educator & Researcher
Independent Educational Consultant • India
Dr. Irfan Mansuri brings 25 years of extensive experience in science education, specializing in Physics and Chemistry. With a doctoral degree and a passion for making science engaging, he has mentored countless students to achieve excellence in their board examinations. His teaching methodology emphasizes conceptual clarity, practical applications, and exam-oriented strategies that help students not just memorize, but truly understand scientific principles.
📚 Related Questions You Might Find Helpful:
- Activity 1.1: Burning of Magnesium Ribbon in Air
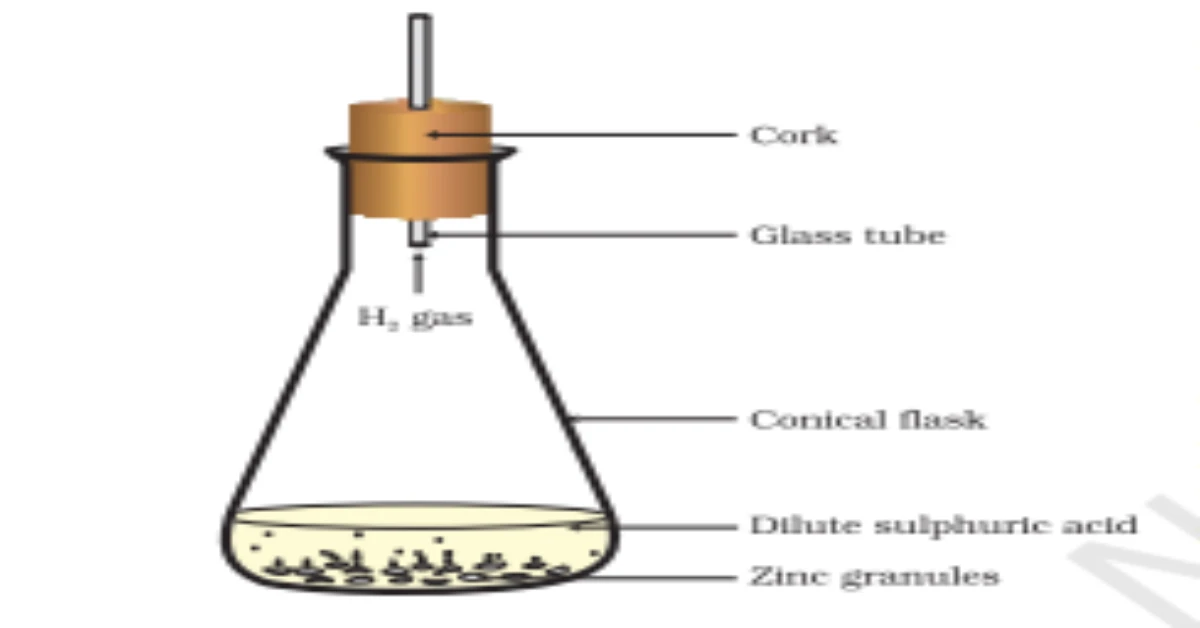
- Activity 1.2: Reaction of Zinc with Dilute Sulphuric Acid
- Activity 1.7: Reaction of Iron Nails with Copper Sulphate Solution
- What are the different types of decomposition reactions?
- How to balance chemical equations step by step?