What Happens During Electrolysis of Water? | Class 10 Science Ch 1 Activity 1.7
Activity 1.7 from your Class 10 Science textbook is one of the most fascinating practical demonstrations of chemical decomposition. This electrolysis experiment shows how electrical energy can break down water molecules into their constituent elements—hydrogen and oxygen. Understanding this activity is crucial not just for your board exams but also for grasping fundamental concepts in electrochemistry that you’ll encounter in higher classes.
EXAM TIP: Looking for the Perfect Answer to Write in Your Exam?
Skip the long explanation and get straight to the exam-ready answer format that will help you score full marks! We’ve prepared the perfect answer structure with proper marking scheme breakdown for this practical activity.
📝 Jump to Exam-Ready Answer (5 Marks) →⚡ Quick Answer: What Happens in Activity 1.7?
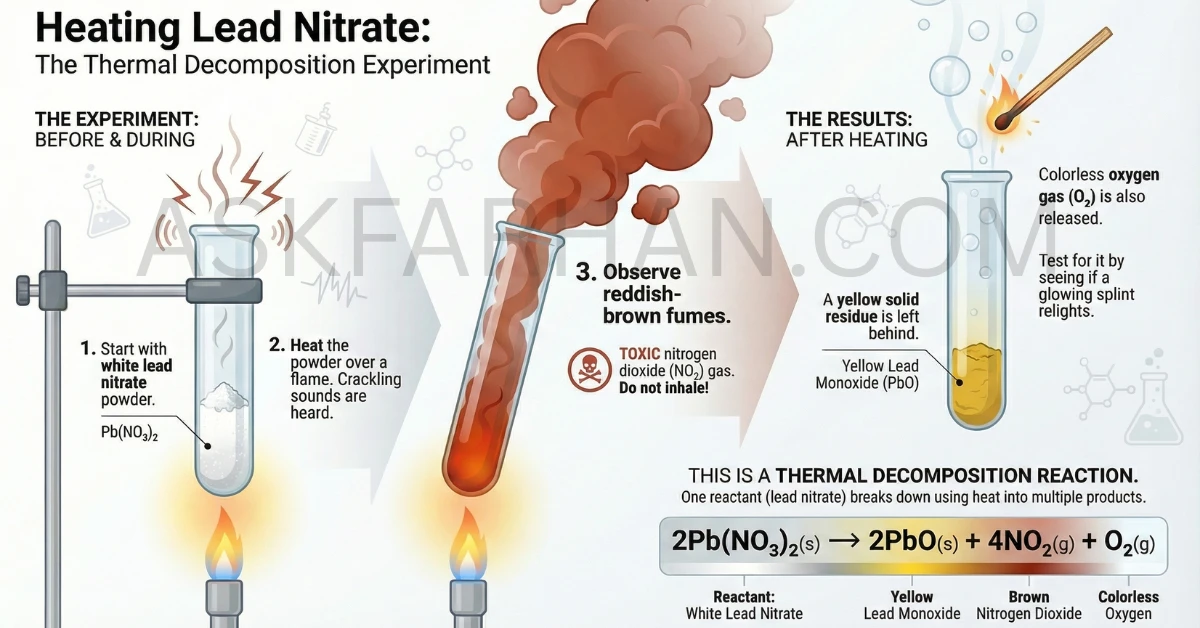
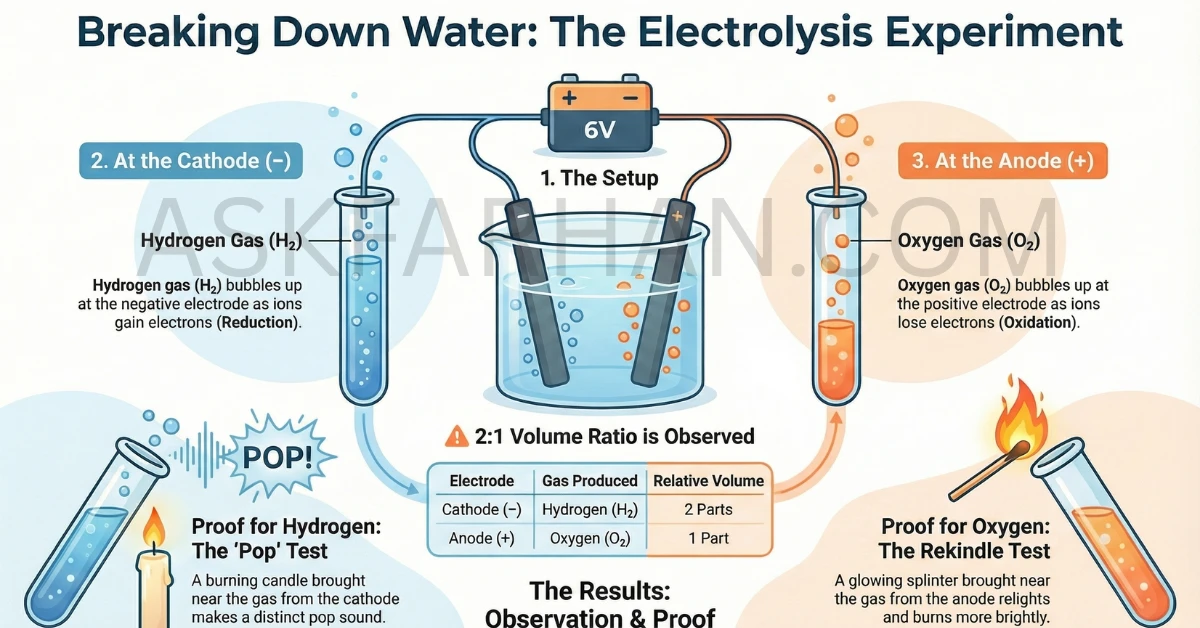
When electric current passes through acidulated water (water + dilute H2SO4), electrolysis occurs. Water molecules decompose into hydrogen gas (H2) and oxygen gas (O2). Hydrogen collects at the cathode (negative electrode) and oxygen at the anode (positive electrode). The volume ratio is approximately 2:1 (hydrogen:oxygen). Hydrogen gas burns with a pop sound when tested with a burning candle, while oxygen gas makes the candle burn more brightly.
🔬 Understanding the Electrolysis of Water
What is Electrolysis?
Electrolysis is a chemical decomposition reaction caused by passing electric current through a liquid or solution containing ions. In this activity, we’re decomposing water (H2O) into its constituent elements—hydrogen (H2) and oxygen (O2).
The word “electrolysis” comes from two Greek words: electro (electricity) and lysis (breaking down). So literally, it means “breaking down using electricity.”
Why Add Dilute Sulphuric Acid?
Pure water is a poor conductor of electricity because it contains very few ions. To make the electrolysis process efficient, we add a few drops of dilute sulphuric acid (H2SO4). The acid dissociates into ions:
H2SO4 → 2H+ + SO42-
These ions make the water conductive, allowing electric current to flow through it. The sulphuric acid itself doesn’t get decomposed—it only acts as an electrolyte (a substance that helps conduct electricity).
🔬 Apparatus Setup and Diagram Description
ELECTROLYSIS OF WATER - APPARATUS SETUP
6V Battery
[+ -]
│ │
│ │
┌──────────┘ └──────────┐
│ │
Test Tube A Test Tube B
(filled with (filled with
water) water)
│ │ │ │
│ │ │ │
↓ ↓ ↓ ↓
┌─────────────────────────────┐
│ ○ ○ │ ← Water level
│ │ │ │
│ │ Acidulated │ │
│ │ Water │ │
│ │ (H₂O + H₂SO₄) │ │
│ │ │ │
│ [─] [+] │ ← Carbon electrodes
│ Cathode Anode │
│ (-) (+) │
└─────────────────────────────┘
Plastic Mug with
rubber stoppers at base
OBSERVATIONS:
• Gas A (at cathode): Hydrogen (H₂) - Volume: 2 parts
• Gas B (at anode): Oxygen (O₂) - Volume: 1 part
• Ratio H₂:O₂ = 2:1
Components Explained:
Provides electrical energy to decompose water molecules
Inert electrodes that don’t react with water or gases produced
Collect gases produced at each electrode (inverted over electrodes)
Water + few drops H2SO4 to make it conductive
⚗️ The Chemical Reaction
The overall chemical equation for the electrolysis of water is:
2H2O(l) → 2H2(g) + O2(g)
Electrical energy
This equation tells us that:
- 2 molecules of water decompose to produce 2 molecules of hydrogen gas and 1 molecule of oxygen gas
- The reaction requires electrical energy to proceed (endothermic decomposition)
- The volume ratio of gases produced is H2:O2 = 2:1
What Happens at Each Electrode?
⊖ At Cathode (Negative Electrode)
Reduction occurs:
2H+ + 2e– → H2↑
Hydrogen ions gain electrons and form hydrogen gas, which bubbles up and collects in the test tube.
⊕ At Anode (Positive Electrode)
Oxidation occurs:
4OH– → 2H2O + O2↑ + 4e–
Hydroxide ions lose electrons and form oxygen gas and water. Oxygen bubbles up and collects in the test tube.
💡 Remember: OIL RIG
Oxidation Is Loss (of electrons) – happens at Anode
Reduction Is Gain (of electrons) – happens at Cathode
🧪 Observations and Gas Tests
Volume of Gases Collected
Question: Is the volume of gas collected the same in both test tubes?
Answer: No, the volumes are different.
The test tube at the cathode collects approximately twice the volume of gas compared to the test tube at the anode. This 2:1 ratio directly reflects the chemical equation:
2 volumes of H2 : 1 volume of O2
Testing the Gases
| Gas | Collected At | Test Performed | Observation | Conclusion |
|---|---|---|---|---|
| Hydrogen (H2) | Cathode (−) | Bring burning candle near mouth of test tube | Pop sound is heard; gas burns with pale blue flame | Hydrogen is present (combustible gas) |
| Oxygen (O2) | Anode (+) | Bring glowing splinter near mouth of test tube | Glowing splinter rekindles (burns more brightly) | Oxygen is present (supports combustion) |
⚠️ Common Mistakes Students Make
❌ Mistake 1: Confusing which gas is collected where
Remember: “HyCat” – Hydrogen at Cathode (both start with consonants). Oxygen is at the Anode.
❌ Mistake 2: Writing wrong volume ratio
Correct ratio: H2:O2 = 2:1 (not 1:2). Look at the chemical equation—2 molecules of H2 are produced for every 1 molecule of O2.
❌ Mistake 3: Forgetting to mention acidulated water
Important: Always mention that dilute H2SO4 is added to water. Pure water won’t conduct electricity efficiently.
🔬 Lab Connection: Practical Tips
Safety Precautions:
- Use only 6V battery (low voltage for safety)
- Handle dilute H2SO4 carefully—it’s corrosive
- Don’t touch electrodes while current is flowing
- Test hydrogen gas carefully—it’s highly flammable
- Ensure proper ventilation in the lab
Tips for Better Results:
- Add only 2-3 drops of H2SO4—too much will make water heat up
- Ensure test tubes are completely filled with water before inverting
- Keep electrodes at least 5 cm apart to avoid short circuit
- Wait patiently—it takes 15-20 minutes to collect sufficient gas
- Use carbon electrodes (from old batteries) as they don’t react
Common Lab Problems:
Problem: Very few bubbles forming
Solution: Add 1-2 more drops of H2SO4 or check battery connections.
Problem: Water getting hot
Solution: Too much acid added or voltage too high. Use fresh water with less acid.
📝 How to Write This Answer in Your Exam (5 Marks)
Marking Scheme Breakdown:
- 1 mark: Apparatus setup and procedure
- 1 mark: Observation about gas volumes
- 1 mark: Chemical equation
- 1 mark: Gas identification tests
- 1 mark: Conclusion about which gas is at which electrode
Model Answer for Exam:
Aim: To demonstrate the electrolysis of water and identify the gases produced.
Procedure: A plastic mug with two carbon electrodes fitted at the base is filled with water. A few drops of dilute sulphuric acid are added to make it conductive. Two test tubes filled with water are inverted over the electrodes. The electrodes are connected to a 6V battery and current is passed.
Observations:
- Bubbles form at both electrodes and rise up into the test tubes
- The volume of gas collected at the cathode is approximately twice the volume collected at the anode
- The ratio of gases is 2:1
Chemical Equation:
2H2O(l) → 2H2(g) + O2(g)
Gas Tests:
- Gas at cathode: When a burning candle is brought near, it burns with a pop sound. This confirms the presence of hydrogen gas (H2).
- Gas at anode: When a glowing splinter is brought near, it rekindles (burns more brightly). This confirms the presence of oxygen gas (O2).
Conclusion: Electrolysis of water produces hydrogen gas at the cathode and oxygen gas at the anode in the volume ratio 2:1, proving that water is composed of hydrogen and oxygen in the ratio H2O.
✍️ Exam Writing Tips:
- Draw a neat labeled diagram if asked (1-2 extra marks possible)
- Always write the balanced chemical equation
- Mention the 2:1 volume ratio clearly
- Describe both gas tests with observations
- Use proper chemical formulas with subscripts
✅ Complete Step-by-Step Solution
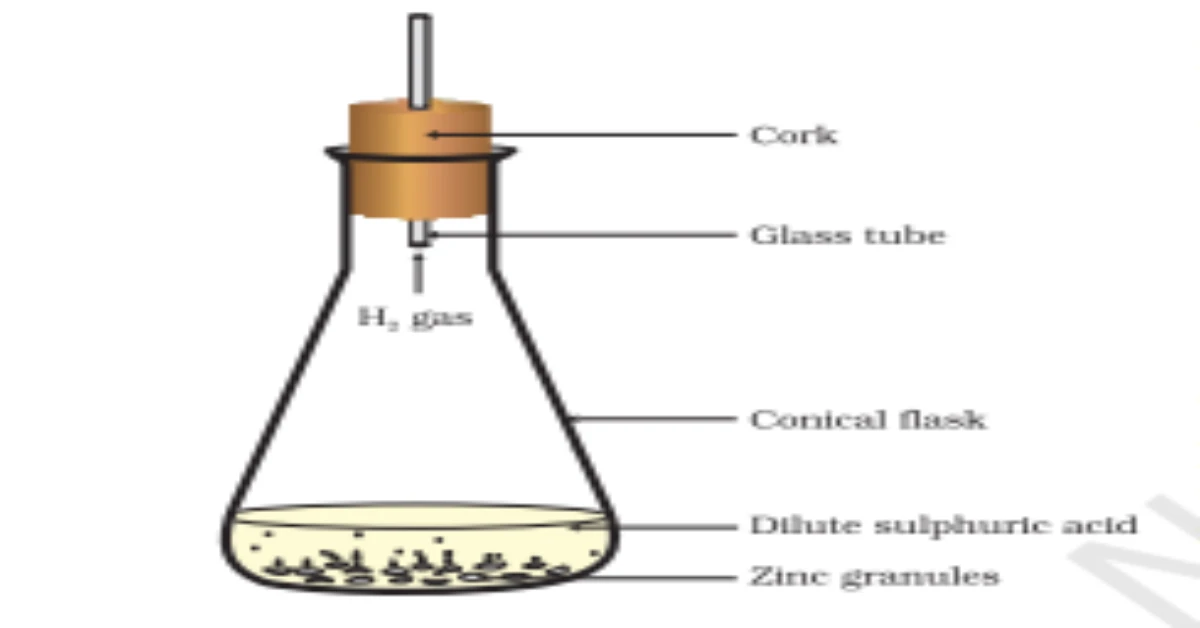
Take a plastic mug and drill two holes at its base. Fit rubber stoppers in these holes and insert carbon electrodes through them. Connect these electrodes to a 6V battery using wires.
Fill the mug with water until the electrodes are completely immersed. Add 2-3 drops of dilute sulphuric acid (H2SO4) to make the water conductive. The acid dissociates into H+ and SO42- ions.
Take two test tubes, fill them completely with water, and invert them over the two carbon electrodes. Ensure no air bubbles are trapped inside the test tubes initially.
Switch on the battery. Electric current flows through the acidulated water, causing electrolysis. Water molecules start decomposing into hydrogen and oxygen gases.
After a few minutes, you’ll see bubbles forming at both electrodes. These bubbles rise up and collect in the inverted test tubes, gradually displacing the water. The cathode test tube fills faster than the anode test tube.
Question: Is the volume of gas collected the same in both test tubes?
Answer: No. The test tube at the cathode collects approximately twice the volume of gas compared to the test tube at the anode. This 2:1 ratio matches the chemical equation.
Carefully remove both test tubes. Test each gas:
• Cathode gas: Bring a burning candle near—it produces a pop sound (hydrogen)
• Anode gas: Bring a glowing splinter near—it rekindles (oxygen)
Hydrogen gas (H2) is collected at the cathode and oxygen gas (O2) is collected at the anode in the volume ratio 2:1. This proves that water (H2O) is composed of hydrogen and oxygen in a 2:1 atomic ratio.
📊 Summary of Results:
Hydrogen (H2)
2 volumes
Oxygen (O2)
1 volume
🌍 Real-World Applications of Electrolysis
The electrolysis process you performed in Activity 1.7 isn’t just a classroom experiment—it has numerous practical applications in industry and everyday life:
Hydrogen Fuel Production
Electrolysis is used to produce hydrogen gas for fuel cells in electric vehicles and space rockets. It’s a clean energy source that produces only water as a byproduct.
Medical Oxygen
Hospitals use electrolysis to produce pure oxygen for patients with respiratory problems. The oxygen produced is of high purity.
Industrial Chemicals
Electrolysis of brine (salt water) produces chlorine gas, hydrogen gas, and sodium hydroxide—all important industrial chemicals.
Electroplating
Electrolysis is used to coat objects with thin layers of metals like gold, silver, or chromium for decoration and corrosion protection.
❓ Frequently Asked Questions (FAQs)
1. Why do we add dilute H2SO4 to water? Can we use other acids?
Pure water is a poor conductor of electricity because it has very few ions. Dilute H2SO4 provides H+ and SO42- ions that make water conductive. We use H2SO4 specifically because it doesn’t get reduced or oxidized during electrolysis—it only acts as an electrolyte. You could theoretically use other acids like HCl, but HCl would produce chlorine gas at the anode instead of pure oxygen, which would interfere with the experiment.
2. Why is the volume of hydrogen twice that of oxygen?
This directly comes from the chemical formula of water: H2O. Each water molecule contains 2 hydrogen atoms and 1 oxygen atom. When water decomposes, it produces 2 molecules of H2 gas for every 1 molecule of O2 gas, as shown in the equation: 2H2O → 2H2 + O2. Since equal numbers of molecules occupy equal volumes under the same conditions (Avogadro’s Law), the volume ratio is also 2:1.
3. What happens if we reverse the battery connections?
If you reverse the battery connections, the polarity of the electrodes will reverse. The electrode that was previously the cathode (producing hydrogen) will become the anode (producing oxygen), and vice versa. The gases will simply be collected at opposite electrodes, but the chemical process and volume ratio remain the same. This proves that the gas production depends on the electrode polarity, not the physical electrode itself.
4. Is electrolysis of water an endothermic or exothermic reaction?
Electrolysis of water is an endothermic reaction. It requires continuous input of electrical energy to break the strong O-H bonds in water molecules. The energy required to break these bonds (bond dissociation energy) is greater than the energy released when new H-H and O=O bonds form. That’s why we need to keep supplying electricity throughout the process—if we stop, the reaction stops immediately.
5. Can we perform electrolysis with salt water instead of acidulated water?
Yes, you can use salt water (NaCl solution) as it also conducts electricity due to Na+ and Cl– ions. However, the products will be different. At the cathode, you’ll still get hydrogen gas, but at the anode, you’ll get chlorine gas (Cl2) instead of oxygen because chloride ions are more easily oxidized than water molecules. This is actually how chlorine gas is produced industrially through the chlor-alkali process. For this specific activity demonstrating water decomposition, acidulated water is preferred to get pure oxygen.
✍️ Written by Dr. Irfan Mansuri
Senior Science Educator & Researcher
Independent Educational Consultant • India
Dr. Irfan Mansuri brings 25 years of extensive experience in science education, specializing in Physics and Chemistry. With a doctoral degree and a passion for making science engaging, he has mentored countless students to achieve excellence in their board examinations. His teaching methodology emphasizes conceptual clarity, practical applications, and exam-oriented strategies that help students not just memorize, but truly understand scientific principles.
📚 Related Questions from Chapter 1
Explore more questions from Chemical Reactions and Equations:
- Activity 1.1: Burning of magnesium ribbon in air
- Activity 1.2: Reaction of lead nitrate with potassium iodide
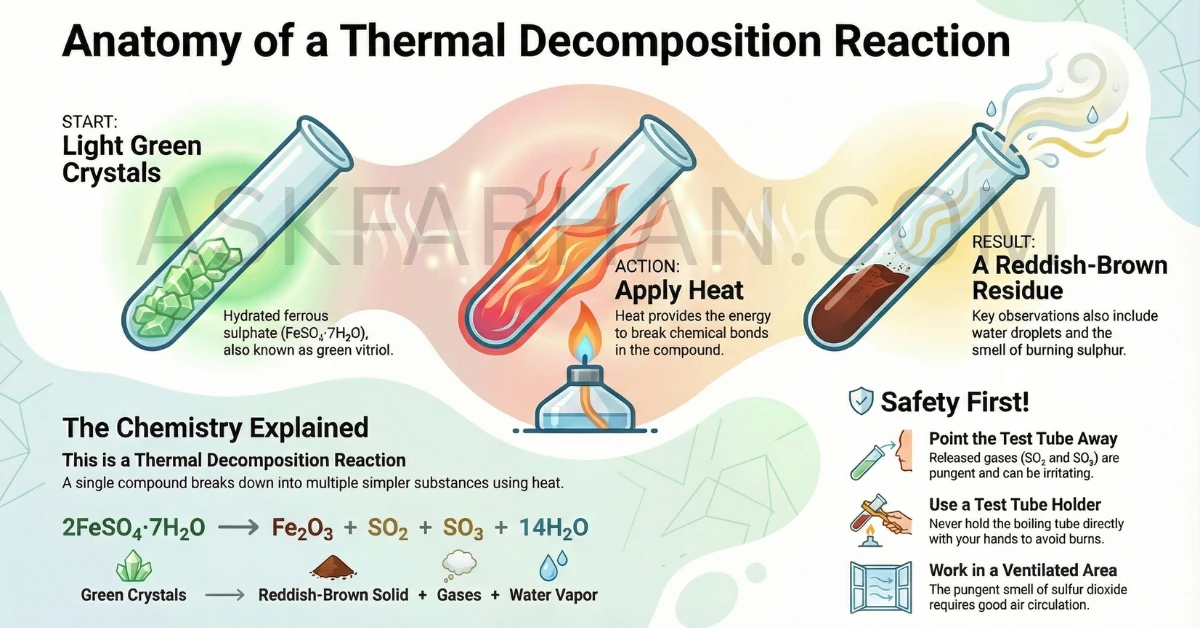
- Activity 1.5: Heating of ferrous sulphate crystals
- Activity 1.10: Displacement reactions with iron nails
- Question 1: Why should a magnesium ribbon be cleaned before burning?