Heating Ferrous Sulphate Crystals – Activity 1.5 | Class 10 Science Ch 1
Activity 1.5 from your NCERT Class 10 Science textbook is a fascinating hands-on experiment that demonstrates thermal decomposition. When you heat those beautiful green ferrous sulphate crystals, you’ll witness an amazing color transformation that reveals the chemistry happening right before your eyes!
EXAM TIP: Looking for the Perfect Answer to Write in Your Exam?
Skip the long explanation and get straight to the exam-ready answer format that will help you score full marks! We’ve prepared the perfect answer structure with proper observation recording and chemical equation.
📝 Jump to Exam-Ready Answer (3 Marks) →⚡ Quick Answer
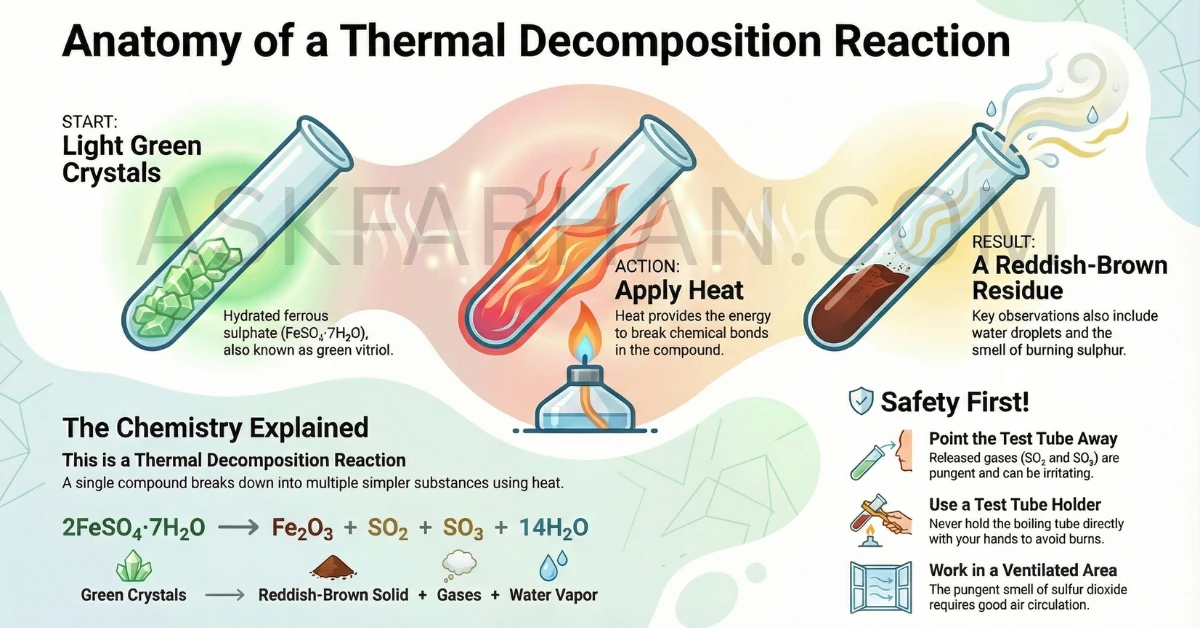
When green ferrous sulphate crystals (FeSO4·7H2O) are heated, they lose water molecules and decompose into reddish-brown ferric oxide (Fe2O3), sulfur dioxide (SO2), and sulfur trioxide (SO3) gases. This is a thermal decomposition reaction where a single compound breaks down into multiple products upon heating.
🔬 Understanding the Activity
This activity is designed to help you observe and understand thermal decomposition reactions. Ferrous sulphate is a hydrated salt, meaning it contains water molecules in its crystal structure. When we apply heat, we’re providing energy that breaks chemical bonds and drives off these water molecules, followed by further decomposition of the compound itself.
What is Ferrous Sulphate?
Ferrous sulphate (FeSO4·7H2O) is also known as “green vitriol” because of its characteristic light green color. The “7H2O” indicates that each formula unit contains seven water molecules attached to it. These water molecules are called “water of crystallization” and are responsible for the crystal structure and color of the compound.
🎨 Visual Representation of Color Change
⚠️ Additional Observations: You’ll also notice the smell of burning sulphur (SO2 gas) and water droplets forming on the cooler parts of the test tube!
✅ Step-by-Step Procedure & Observations
Take approximately 2 grams of ferrous sulphate crystals in a clean, dry boiling tube. Make sure the tube is completely dry as moisture can affect the observations.
Carefully observe and note the color of the ferrous sulphate crystals. They should appear light green or pale green in color. This is your “before heating” observation.
Hold the boiling tube with a test tube holder at an angle (about 45°) and heat it gently over a burner or spirit lamp flame. Keep the mouth of the tube pointing away from yourself and others. Heat continuously for 3-5 minutes.
Watch carefully! You’ll notice: (a) Water droplets forming on the cooler upper parts of the tube, (b) The green color gradually fading, (c) A characteristic smell of burning sulphur (SO2 gas), (d) The crystals turning brown/reddish-brown.
After heating, let the tube cool down. The final residue will be reddish-brown or brown in color. This is ferric oxide (Fe2O3). The color change from green to brown is your key observation!
⚗️ The Chemical Reaction Explained
The heating of ferrous sulphate is a perfect example of a thermal decomposition reaction. This type of reaction occurs when a single compound breaks down into two or more simpler substances upon heating.
📋 Chemical Equation
Breaking it down: Two molecules of hydrated ferrous sulphate decompose to form one molecule of ferric oxide (solid), one molecule of sulfur dioxide gas, one molecule of sulfur trioxide gas, and fourteen molecules of water (steam).
🔍 What’s Happening at the Molecular Level?
When you apply heat, you’re providing energy to break the chemical bonds in ferrous sulphate. First, the water of crystallization evaporates (you see water droplets). Then, the anhydrous ferrous sulphate (FeSO4) further decomposes into ferric oxide, releasing sulfur dioxide and sulfur trioxide gases. The iron changes its oxidation state from +2 (ferrous) to +3 (ferric), which explains the color change from green to reddish-brown.
⚠️ Safety Precautions & Common Mistakes
🛡️ Safety First!
- Always use a test tube holder – Never hold the tube directly with your hands
- Point the mouth away – Keep the tube opening away from yourself and others
- Work in ventilated area – SO2 gas has a pungent smell and can irritate
- Wear safety goggles – Protect your eyes from any splashes
- Don’t overheat – Gentle heating is sufficient; overheating may crack the tube
- Let it cool naturally – Don’t touch the hot tube immediately after heating
❌ Common Mistakes Students Make
Using a wet test tube – This dilutes the crystals and affects observations. Always use a dry tube!
Not recording the initial color properly – Write down “light green” or “pale green” before heating.
Stopping heating too early – Heat continuously for 3-5 minutes to see complete decomposition.
🌍 Real-World Applications
Thermal decomposition reactions like this one have several practical applications in our daily lives and industries:
Industrial Manufacturing
Decomposition of limestone (CaCO3) to produce lime (CaO) for cement manufacturing uses similar thermal decomposition principles.
Medicine Production
Ferric oxide produced from ferrous sulphate decomposition is used in iron supplements and as a pigment in pharmaceutical coatings.
Pigment Industry
The reddish-brown ferric oxide is widely used as a pigment in paints, cosmetics, and coloring materials.
📝 How to Write This Answer in Your Exam (3 Marks)
🎯 Perfect Exam Answer Format
Aim: To observe the decomposition of ferrous sulphate on heating.
Procedure: About 2 g of ferrous sulphate crystals are taken in a dry boiling tube and heated over a burner flame.
Observations:
• Initial color: Light green (ferrous sulphate crystals)
• After heating: Reddish-brown residue (ferric oxide)
• Water droplets appear on cooler parts of tube
• Smell of burning sulphur (SO2 gas) is observed
Chemical Equation:
2FeSO4·7H2O → Fe2O3 + SO2 + SO3 + 14H2O
Conclusion: This is a thermal decomposition reaction where ferrous sulphate decomposes into ferric oxide, sulfur dioxide, sulfur trioxide, and water on heating.
💡 Marking Scheme Tip: You get 1 mark for observations, 1 mark for the correct chemical equation, and 1 mark for identifying it as a decomposition reaction. Always write the color change clearly!
🎥 Video Explanation
Watch this detailed video explanation to see the actual experiment being performed with step-by-step guidance and visual demonstrations of the color change!
❓ Frequently Asked Questions (FAQs)
❓ Why does the color change from green to brown when ferrous sulphate is heated?
The color change occurs because the chemical composition changes. Green ferrous sulphate (containing Fe2+ ions) decomposes into reddish-brown ferric oxide (containing Fe3+ ions). The oxidation state of iron changes from +2 to +3, which results in the dramatic color transformation. Think of it like iron rusting – fresh iron is grayish, but rust (iron oxide) is brown!
❓ What is the smell observed during this experiment?
The characteristic pungent smell is from sulfur dioxide (SO2) gas, which is released during the decomposition. It smells like burning sulphur or burnt matchsticks. This is why the experiment should be performed in a well-ventilated area. SO2 is the same gas that causes acid rain when released in large quantities into the atmosphere.
❓ Why do water droplets form on the cooler parts of the test tube?
Ferrous sulphate is a hydrated salt containing water of crystallization (7H2O). When heated, this water is released as steam. As the steam rises and touches the cooler upper parts of the test tube, it condenses back into liquid water, forming visible droplets. This proves that the original compound contained water molecules in its crystal structure.
❓ Is this a reversible reaction? Can we get back green ferrous sulphate?
No, this is an irreversible reaction. Once ferrous sulphate decomposes into ferric oxide, SO2, SO3, and water, you cannot simply reverse the process by cooling. The gases escape into the atmosphere, and the chemical bonds have been permanently broken. To make ferrous sulphate again, you would need to perform completely different chemical reactions starting from iron and sulfuric acid.
❓ What type of chemical reaction is this, and why is it important?
This is a thermal decomposition reaction (also called thermolysis). It’s a type of decomposition reaction where heat energy is used to break down a single compound into multiple simpler substances. This type of reaction is extremely important in industries – for example, the production of quicklime (CaO) from limestone (CaCO3) for cement manufacturing, and the extraction of metals from their ores all involve thermal decomposition reactions.
✍️ Written by Dr. Irfan Mansuri
Senior Science Educator & Researcher
Independent Educational Consultant • India
Dr. Irfan Mansuri brings 25 years of extensive experience in science education, specializing in Physics and Chemistry. With a doctoral degree and a passion for making science engaging, he has mentored countless students to achieve excellence in their board examinations. His teaching methodology emphasizes conceptual clarity, practical applications, and exam-oriented strategies that help students not just memorize, but truly understand scientific principles.
📚 Related Questions from Chapter 1
- Activity 1.1: Burning of Magnesium Ribbon
- Activity 1.2: Reaction of Lead Nitrate with Potassium Iodide
- Activity 1.3: Zinc and Dilute Sulphuric Acid Reaction
- Activity 1.4: Iron Nails in Copper Sulphate Solution
- What is a Balanced Chemical Equation?
Note: Internal linking URLs to be added by website administrator