What is Electrolysis? | Class 10 Chemistry Explained
What is Electrolysis? | Class 10 Chemistry Explained
Have you ever wondered how we extract pure metals from their compounds or coat jewelry with gold? The answer lies in a fascinating chemical process called electrolysis! This process uses electricity to drive chemical reactions that wouldn’t happen on their own. Understanding electrolysis is crucial for your Class 10 Chemistry exams and helps explain many industrial processes around us. Let’s explore this concept in simple, easy-to-understand terms.
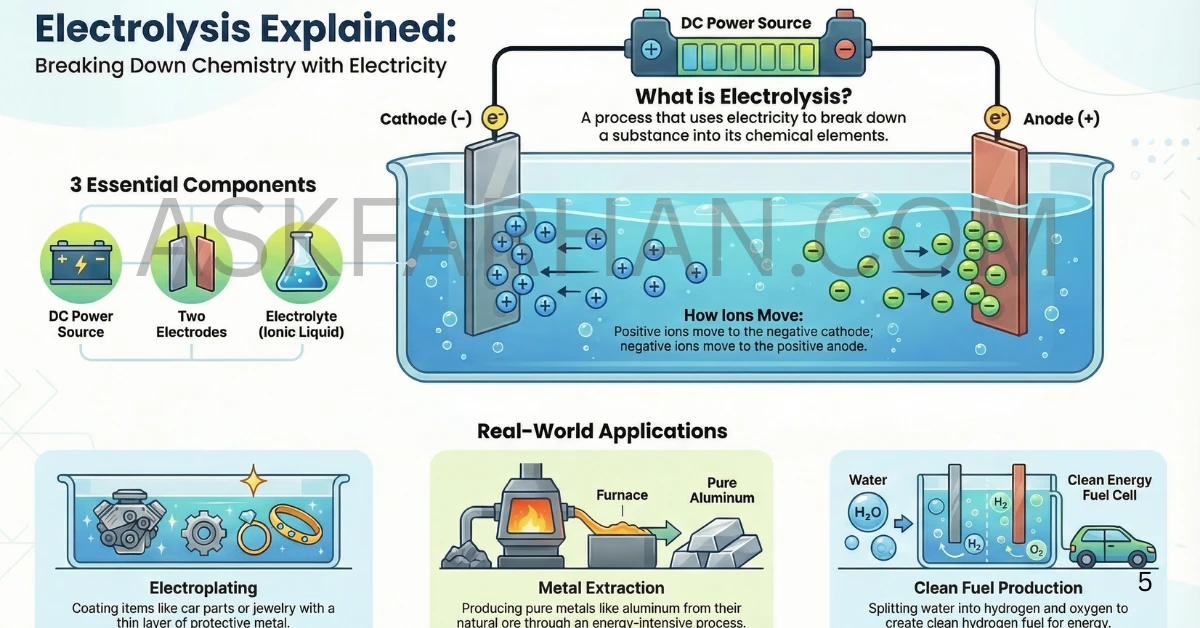
Definition of Electrolysis
Electrolysis is the process of using electric current to bring about a chemical decomposition or chemical change in a substance. In simpler terms, it’s breaking down chemical compounds into their elements by passing electricity through them. The word “electrolysis” comes from “electro” (electricity) and “lysis” (breaking down).
How Does Electrolysis Work? 🔬
The process of electrolysis requires three essential components working together. First, you need an electrolyte – a liquid or solution that conducts electricity, usually containing ions. Second, you need two electrodes – the anode (positive electrode) and cathode (negative electrode) – which are typically made of metal or graphite. Finally, you need a source of direct current (DC) like a battery.
When electric current passes through the electrolyte, positive ions (cations) move toward the cathode, where they gain electrons in a process called reduction. Meanwhile, negative ions (anions) move toward the anode, where they lose electrons through oxidation. This movement of ions completes the electrical circuit and causes chemical changes at both electrodes.
The Electrolysis Setup
Battery (+) ————————— (–)
| |
| |
ANODE CATHODE
| |
| |
└──── Electrolyte ────┘
(Ionic Solution)
• Anions (–) → Move to Anode (+)
• Cations (+) → Move to Cathode (–)
Key Points to Remember 📌
- Electrolysis requires direct current (DC) – alternating current won’t work because ions need to move in one consistent direction.
- Oxidation occurs at the anode – this is where negative ions lose electrons and get oxidized.
- Reduction happens at the cathode – positive ions gain electrons here and get reduced.
- The electrolyte must contain free-moving ions – solid ionic compounds won’t conduct electricity; they must be molten or dissolved.
- Energy is consumed in electrolysis – unlike batteries that produce electricity, electrolysis uses electrical energy to drive non-spontaneous reactions.
Real-World Examples 💡
Electroplating: Ever seen shiny chrome-plated car parts or gold-plated jewelry? That’s electrolysis in action! A thin layer of one metal is deposited onto another object by making it the cathode in an electrolytic cell. This protects the base metal from corrosion and improves appearance.
Extraction of Aluminum: Aluminum is extracted from its ore (bauxite) through electrolysis. The process involves passing electricity through molten aluminum oxide, separating pure aluminum metal at the cathode. This is why aluminum production requires enormous amounts of electricity.
Water Purification: Electrolysis of water produces hydrogen gas at the cathode and oxygen gas at the anode. This process is used to generate clean hydrogen fuel and is being explored as a renewable energy solution.
💡 Did You Know? The same principle of electrolysis is used in rechargeable batteries! When you charge your phone or laptop, you’re essentially reversing a chemical reaction through electrolysis, storing energy that can be released later.
Why Electrolysis Matters
Electrolysis isn’t just a classroom concept – it’s a fundamental process that powers modern industry and technology. From producing essential chemicals like chlorine and sodium hydroxide to enabling green hydrogen production for clean energy, electrolysis plays a vital role in our daily lives. Understanding this process helps you appreciate how chemistry and electricity work together to transform materials and create the products we use every day.
✍️ Written by Dr. Irfan Mansuri
Senior Science Educator & Researcher
Dr. Irfan Mansuri brings 25 years of science education expertise, specializing in making scientific concepts clear and engaging for students. His teaching emphasizes conceptual understanding and practical applications.
Connect on LinkedIn