NCERT Solutions for Class 10 Science Chapter 1 Activity 1.1
Understanding the Question 🧐
This is a fundamental ncert solutions for class 10 science activity that introduces us to one of the most important concepts in chemistry: the particulate nature of matter. When we dissolve salt or sugar in water, we observe an interesting phenomenon that seems magical at first – the solid substance appears to “disappear”! But where does it go? Does it really vanish into thin air?

This activity helps us understand that matter is made up of tiny particles that are too small to be seen with our naked eyes. These particles have spaces between them, and when we dissolve one substance in another, the particles of the dissolved substance occupy these spaces. This is a cornerstone concept in understanding the nature of matter in class 10 science chapter 1.
📝 Activity 1.1: Observing the Dissolution of Salt/Sugar in Water
Procedure:
Questions to Answer:
- What do you think has happened to the salt?
- Where does it disappear?
- Does the level of water change?
Detailed Explanation 🧪
🔍 What Happens to the Salt?
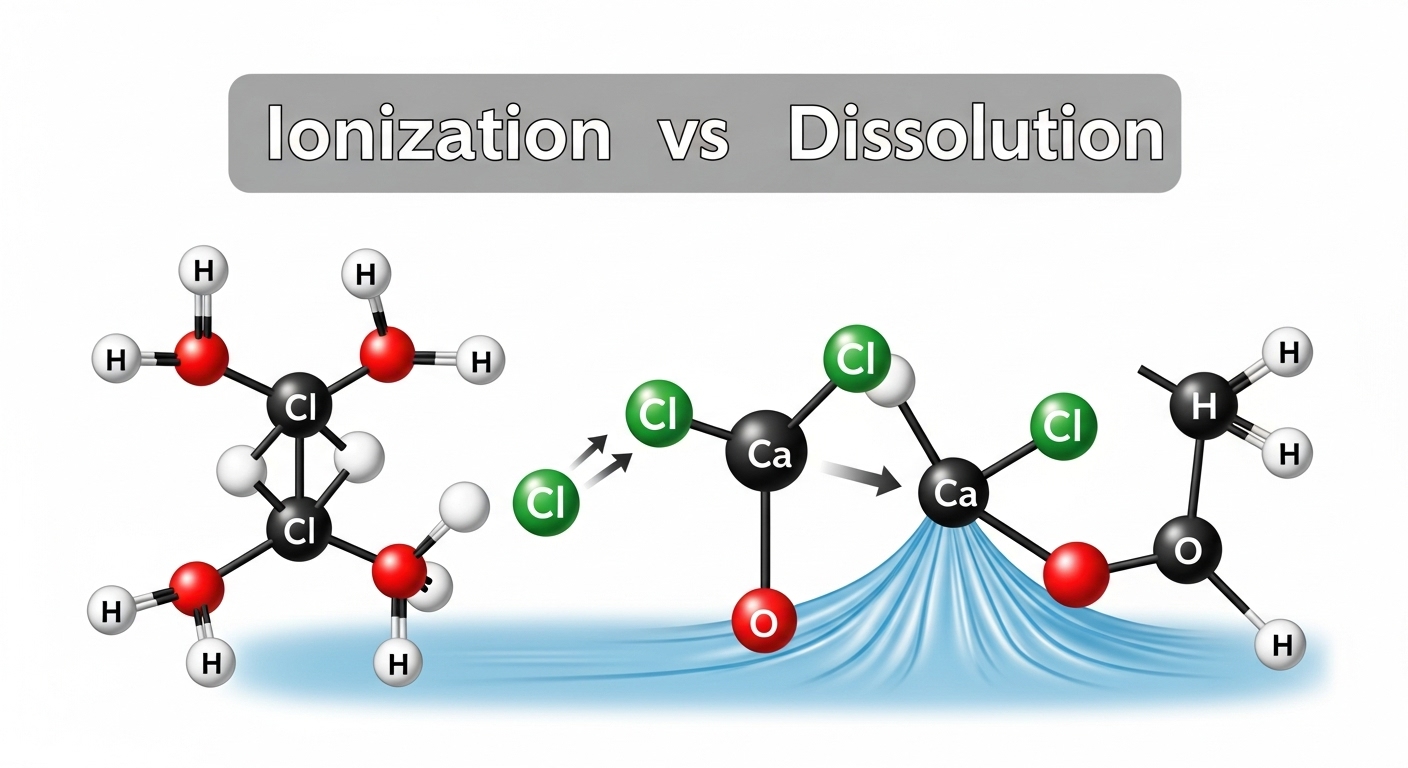
When you add salt (&&\ce{NaCl}&&) or sugar (&&\ce{C12H22O11}&&) to water and stir it with a glass rod, you’ll notice that the solid crystals gradually become smaller and smaller until they are no longer visible. The salt doesn’t actually disappear – it’s still there in the water!
Here’s what’s really happening:
The salt crystals are made up of millions of tiny particles (ions in the case of salt: &&\ce{Na+}&& and &&\ce{Cl-}&&). When salt comes in contact with water, these particles separate from each other and spread throughout the water. Think of it like this: imagine a crowded room with people standing close together (the salt crystals), and then they all spread out evenly across a much larger hall (the water).
🌊 Where Does the Salt Disappear?
The salt particles don’t disappear; they occupy the spaces between water particles. This is the key concept! Water, like all matter, is made up of tiny particles called molecules (&&\ce{H2O}&&). These water molecules are not packed tightly together – there are spaces between them.
When salt dissolves:
- The salt particles (&&\ce{Na+}&& and &&\ce{Cl-}&& ions) break away from the crystal structure
- These particles move into the intermolecular spaces between water molecules
- The salt particles become uniformly distributed throughout the water
- The solution becomes homogeneous (the same throughout)
📏 Does the Water Level Change?
This is the most interesting observation! When you dissolve salt in water, you’ll notice that the water level does not change significantly or changes very slightly. This seems strange at first – we added something to the water, so shouldn’t the level go up?
The scientific explanation:
The water level doesn’t rise much because the salt particles fit into the empty spaces (intermolecular spaces) between water molecules. It’s like filling a jar full of large marbles with sand – the sand grains fit into the spaces between the marbles without significantly increasing the total volume.
Mathematically, if the initial volume of water is &&V_{\text{water}}&&, and we add salt with volume &&V_{\text{salt}}&&, the final volume is:
&&V_{\text{final}} \approx V_{\text{water}} + \text{(very small increase)}&&
The final volume is much less than &&V_{\text{water}} + V_{\text{salt}}&&, because the salt particles occupy intermolecular spaces rather than adding to the total volume.
The Particulate Nature of Matter 🔬
This activity demonstrates the fundamental principle that matter is particulate in nature. This means:
| Property | Explanation |
|---|---|
| Matter is made of particles | All matter (solid, liquid, gas) is composed of tiny particles like atoms, molecules, or ions |
| Particles have spaces between them | These intermolecular spaces allow particles of one substance to fit between particles of another |
| Particles are in constant motion | The particles possess kinetic energy and are always moving (more on this in later activities) |
| Particles attract each other | There are forces of attraction between particles (intermolecular forces) |
In this activity, we’ve directly observed the first two properties: matter is made of particles, and these particles have spaces between them.
Conclusion and Key Principles ✅
Summary of Observations:
- ✓ Salt/sugar crystals dissolve in water and become invisible to the naked eye
- ✓ The dissolved substance spreads uniformly throughout the water
- ✓ The water level remains almost the same after dissolution
- ✓ The solution tastes salty/sweet throughout, confirming uniform distribution
Scientific Conclusion:
This ncert solutions for class 10 science chapter 1 activity proves that matter is made up of tiny particles with spaces between them. When salt dissolves in water, the salt particles (&&\ce{Na+}&& and &&\ce{Cl-}&&) occupy the intermolecular spaces between water molecules (&&\ce{H2O}&&), which is why the water level doesn’t change significantly and the salt seems to “disappear.”
- Particulate Nature of Matter: All matter is composed of tiny particles (atoms, molecules, or ions) that are too small to be seen with the naked eye.
- Intermolecular Spaces: There are spaces between particles of matter. These spaces allow particles of one substance to occupy the spaces between particles of another substance.
- Dissolution: When salt (&&\ce{NaCl}&&) or sugar (&&\ce{C12H22O11}&&) dissolves in water (&&\ce{H2O}&&), the particles separate and spread uniformly throughout the water.
- Volume Change: The water level doesn’t change significantly because dissolved particles occupy intermolecular spaces rather than adding to the volume.
- Homogeneous Mixture: A solution is a homogeneous mixture where the dissolved substance (solute) is uniformly distributed in the dissolving medium (solvent).
- Physical Change: Dissolution is a physical change – the chemical composition of salt and water remains unchanged, and salt can be recovered by evaporation.
Frequently Asked Questions (FAQ) ❓
Why does salt disappear when dissolved in water?
Salt doesn’t actually disappear – it’s still present in the water! When salt (&&\ce{NaCl}&&) dissolves, it breaks down into tiny particles (&&\ce{Na+}&& and &&\ce{Cl-}&& ions) that are too small to see with the naked eye. These particles spread throughout the water by occupying the spaces between water molecules (&&\ce{H2O}&&). This demonstrates the particulate nature of matter – all matter is made up of tiny particles with spaces between them.
Does the water level change when salt is dissolved in it?
No, the water level does not change significantly when salt is dissolved. This is because the salt particles fit into the intermolecular spaces between water particles rather than adding to the overall volume. If the initial water volume is &&V_{\text{water}}&&, the final volume after adding salt is approximately &&V_{\text{water}}&&, not &&V_{\text{water}} + V_{\text{salt}}&&. This observation provides strong evidence for the existence of spaces between particles of matter.
What does this activity tell us about the nature of matter?
This activity demonstrates that matter is particulate in nature. It shows us two fundamental properties: (1) All matter is made up of tiny particles – in this case, salt consists of &&\ce{Na+}&& and &&\ce{Cl-}&& ions, and water consists of &&\ce{H2O}&& molecules. (2) These particles have spaces between them – the salt particles can occupy the intermolecular spaces in water, which is why the water level doesn’t rise significantly. This is a foundational concept in class 10 science for understanding the behavior of matter.
Can we use sugar instead of salt in this activity?
Yes, absolutely! Sugar (&&\ce{C12H22O11}&&) can be used instead of salt (&&\ce{NaCl}&&), and you’ll observe the same phenomenon. Both substances will dissolve in water, and their particles will occupy the spaces between water particles. The water level will remain approximately the same in both cases. The only difference is that sugar dissolves as molecules (&&\ce{C12H22O11}&&), while salt dissolves as ions (&&\ce{Na+}&& and &&\ce{Cl-}&&). Both demonstrate the particulate nature of matter equally well.
What are the spaces between particles called?
The spaces between particles are called intermolecular spaces or interparticle spaces. These spaces exist in all states of matter (solid, liquid, and gas), though their size varies. In gases, the intermolecular spaces are very large; in liquids, they are smaller; and in solids, they are the smallest. During dissolution, particles of the solute (like &&\ce{NaCl}&&) occupy the intermolecular spaces in the solvent (like &&\ce{H2O}&&), which is why the volume doesn’t increase proportionally.
Why do we need to stir with a glass rod?
Stirring with a glass rod serves an important purpose: it speeds up the dissolution process. When we stir, we help distribute the salt particles throughout the water more quickly by increasing the contact between salt and water particles. Without stirring, dissolution would still occur, but it would take much longer because it would rely only on the natural movement of particles (diffusion). Stirring also helps break up any clumps of salt, exposing more surface area to water molecules, which accelerates the rate at which &&\ce{Na+}&& and &&\ce{Cl-}&& ions separate and spread through the water.
What is the particulate nature of matter?
The particulate nature of matter is a fundamental concept in chemistry that states: All matter is composed of extremely small particles (atoms, molecules, or ions) that are too tiny to be seen with the naked eye. These particles have the following characteristics: (1) They are in constant motion and possess kinetic energy. (2) They have spaces between them (intermolecular spaces). (3) They attract each other with varying forces (intermolecular forces). (4) The arrangement and motion of these particles determine the state of matter (solid, liquid, or gas). This activity with salt and water beautifully demonstrates this concept by showing how salt particles (&&\ce{Na+}&& and &&\ce{Cl-}&&) can occupy spaces between water particles (&&\ce{H2O}&&).
Is dissolving salt in water a physical or chemical change?
Dissolving salt in water is a physical change, not a chemical change. Here’s why: In a physical change, no new substance is formed, and the change is usually reversible. When &&\ce{NaCl}&& dissolves in &&\ce{H2O}&&, the salt particles separate and spread throughout the water, but the chemical composition of both salt and water remains unchanged. The &&\ce{Na+}&& and &&\ce{Cl-}&& ions are still present, just dispersed in water. We can recover the original salt by evaporating the water, proving the change is reversible. If it were a chemical change, we would form a new substance with different properties, and the change would be difficult or impossible to reverse.
Further Reading 📚
For more information on the particulate nature of matter and related concepts, you can visit the official NCERT website:
You can also explore more ncert solutions for class 10 science activities and questions to deepen your understanding of matter and its properties.